Abstract
Background: ITP is an acquired autoimmune disorder. Second-line treatment options include EPAG, romiplostim (both stimulate platelet production), ritux (a monoclonal anti-CD20 antibody that causes temporary but complete depletion of peripheral B cells), splenectomy (spl), and other immunosuppressive agents. This subanalysis of EXTEND, a global, open-label, extension study, evaluated long-term EPAG in adults with ITP of ≥6 months' duration according to prior ritux use.
Aims: Describe efficacy and safety of EPAG in ITP patients (pts) according to prior ritux use.
Methods: In EXTEND (Wong et al. Blood 2017;130:2527-36), pts started EPAG at 50 mg/day, with dose adjusted as required to achieve platelet counts of 50-200x109/L.
Results: Of 302 pts in EXTEND, 70 (23%) had previously received ritux. Of these pts, mean±SD age was 53±14 years (yrs), median (range) time since diagnosis was 71.3 (12-362) months, 67% were splenectomized, 61% were female, 76% had baseline platelet counts <30x109/L, 100% had received prior ITP therapy (66% received ≥5 prior therapies), and median (range) time since last ritux was 1.6 (52 days-5.1 yrs) yrs. Of 232 (77%) pts who had not received prior ritux, mean±SD age was 48±16 yrs, median (range) time since diagnosis was 51.6 (9-552) months, 29% were splenectomized, 68% were female, 68% had platelet counts <30x109/L, and 92% had received prior ITP treatment (13% received ≥5 prior therapies).
Mean (range) EPAG daily dose was 53.4 (11-75) mg/day with prior ritux and 49.2 (1-75) mg/day with no prior ritux; median (range) exposure duration was 2.1 (2 days-6.3 yrs) yrs and 2.4 (14 days-8.7 yrs) yrs, respectively.
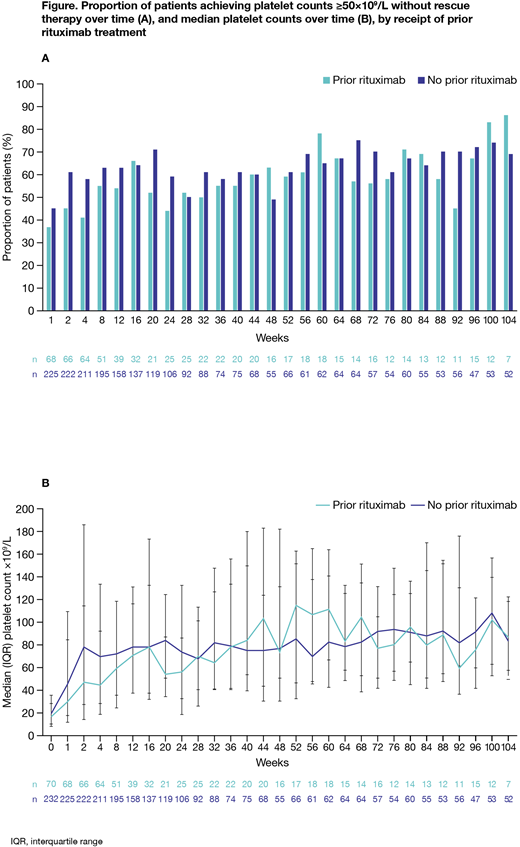
For pts with and without prior ritux, platelet counts were ≥50x109/L without rescue therapy in 37% and 45% at week 1, 45% and 61% at week 2, 41% and 58% at week 4, and 54% and 63% at week 12, respectively; thereafter, the proportion with platelets ≥50x109/L without rescue therapy and median platelet counts were generally similar in both groups (Figure). Estimated median (95% CI) time to platelet counts ≥50 and ≥100x109/L, respectively, was 3.1 (2.1, 6.1) and 8.1 (3.3, 22.4) weeks with prior ritux, and 2.1 (2.0, 2.3) and 4.4 (3.1, 6.1) weeks with no prior ritux. Response to EPAG within the first 2 weeks of treatment seemed more likely in pts with >1.6 yrs since last ritux (n=23; 66%) than in those with ≤1.6 yrs since last ritux (n=14; 40%). Neither the number of previous ITP therapies nor spl status seemed to affect response to EPAG, irrespective of prior ritux.
On-treatment adverse events (AEs) were reported in 68 (97%) and 209 (90%) pts with and without prior ritux, respectively: the most frequent were nasopharyngitis (36%, 21%), headache (33%, 27%), fatigue (29%, 13%), diarrhea (23%, 13%), arthralgia (21%, 13%), and upper respiratory tract infection (URTI; 20%, 24%). Grade 3 AEs occurred in 25 (36%) and 53 (23%) pts, most commonly pain in extremity (6%, <1%) and fatigue (4%, <1%). Serious AEs occurred in 22 (31%) and 74 (32%) pts, most frequently cataract (4%, 6%). The most common AEs considered related to EPAG were headache (13%, 9%), nausea (7%, 3%), fatigue (6%, 4%), cataract (4%, 5%) and increased alanine aminotransferase and aspartate aminotransferase (each 3%, 6%). Bleeding AEs were observed in 24 (34%) and 60 (26%) pts. Overall, incidence of AEs appeared unaffected by spl status. For pts with prior ritux, headache, URTI, pyrexia, and abdominal pain appeared to be more common in pts with <1 yr since last ritux dose, while influenza, epistaxis, rash, anemia, bronchitis and cataract appeared to be more frequent in pts with ≥1 yr since last dose (each ≥10% difference).
Summary/conclusions: EPAG induced similar sustained on-treatment platelet increases in pts with and without prior ritux. While time since ritux did not appear to affect response to EPAG, the proportion of pts with platelet counts ≥50x109/L during the first 12 weeks was higher, albeit not significantly, in the no prior ritux group, despite both cohorts receiving similar EPAG doses. This may pertain to prior ritux pts having harder-to-treat disease (having received more prior ITP treatments, including spl); despite this, EPAG showed similar efficacy in both groups. Further study is required to better understand the higher incidence (≥10%) of the most commonly occurring (eg fatigue, nasopharyngitis and diarrhea) and of the Grade 3 AEs in the prior ritux group, as well as the increased frequency of certain AEs according to time since prior ritux.
Meyer:Amgen, Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bussel:Uptodate: Honoraria; Rigel: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Momenta: Consultancy; Prophylix: Consultancy, Research Funding; Protalex: Consultancy; Amgen Inc.: Consultancy, Research Funding. Wong:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Stankovic:Novartis: Employment. Maier:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal